SteriLux has received ISO 13485:2016 certification
- Press release
- May 09, 2018
- 1min read
News & Calendar SteriLux has received ISO 13485:2016 certification
Prilly, May 9, 2018
We are excited to announce that SteriLux has received ISO 13485:2016 certification for Medical Device and Quality Management Systems from Lloyd’s Register. This ISO certification indicates that the company’s quality management system meets the most current regulatory requirements specific to the medical device industry.
“We are very proud to have received this certification which demonstrates our commitment to quality and is a key step to obtaining the CE marking approval of our product” explains Lucas Meyer, CTO of SteriLux and in charge of quality management.
ISO 13485:2016 is an internationally recognized quality standard for a quality system to ensure customer and regulatory requirements are consistently met throughout the lifecycle of the medical device product.
private
Field manager
Related News All news
-

Contract with AniCura
SteriLux is now the recommended low temperature solution for the AniCura group. This partnership has been in the making for a couple ... + Read more
- Jan 29, 2026
- 1min read
-
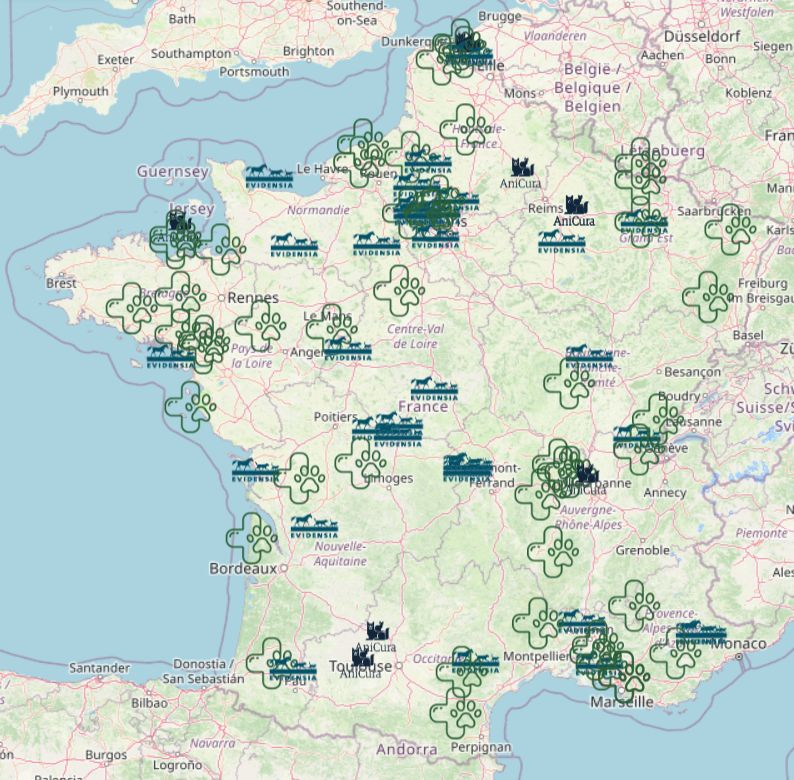
Major Milestone for SteriLux
🎉 Une étape majeure pour SteriLux ! 🇫🇷🐾 Nous sommes très fiers d’annoncer que SteriLux vient de livrer sa 100ᵉ clinique vétérinaire ... + Read more
- Jan 26, 2026
- 2min read
